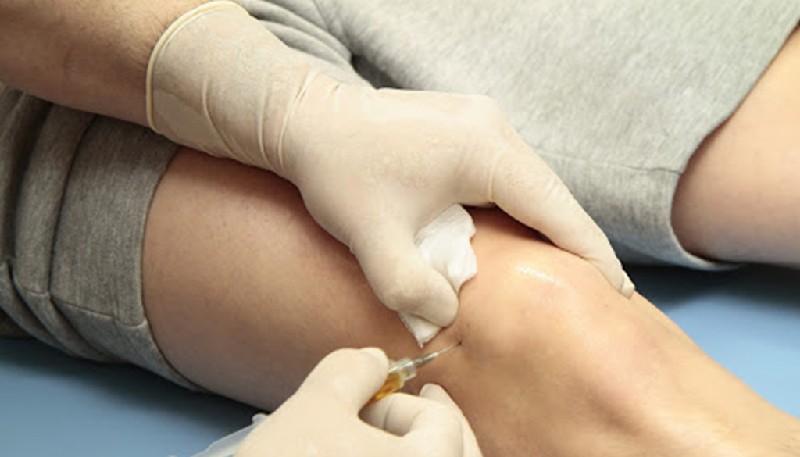
As a healthcare professional with over two decades of experience, I’ve witnessed the evolution of treatment options for osteoarthritis (OA), a degenerative joint disease affecting millions worldwide. While traditional approaches like physical therapy and pain medication remain crucial, the emergence of stem cell therapy has sparked considerable interest. Let’s delve into the potential of stem cells for OA, navigate the current scientific landscape, and explore practical considerations for patients.
Understanding Stem Cells and Their Potential:
Stem cells are the body’s master cells, possessing the remarkable ability to transform into specialized cell types. In the context of OA, mesenchymal stem cells (MSCs) derived from bone marrow or fat tissue hold particular promise. These MSCs, when injected into the affected joint, have the potential to:
The Current Landscape of Research:
It’s important to acknowledge that stem cell therapy for OA is still in its early stages. While some studies have shown positive results, including pain relief and improved function, others have yielded inconclusive evidence [4, 5]. Rigorously designed clinical trials with larger patient populations are needed to definitively establish the efficacy and safety of this approach.
Practical Considerations for Patients:
The regulatory environment surrounding stem cell therapy is complex and varies by region. Currently, in the United States, the Food and Drug Administration (FDA) does not approve Treating Osteoarthritis With stem cell injections for OA treatment outside of approved clinical trials [6]. Patients considering stem cell therapy should be aware of these limitations and the potential risks associated with unproven or unregulated clinics.
Looking Forward: The Future of Stem Cell Therapy for OA
Despite the current limitations, the potential of stem cell therapy for OA remains exciting. Continued research, particularly well-designed clinical trials, is crucial for establishing clear guidelines and protocols for safe and effective use. Additionally, advancements in stem cell isolation, processing techniques, and delivery methods offer promising avenues for future development.
Conclusion:
Stem cell therapy holds promise for the future of OA treatment. However, measured optimism and a critical understanding of the current scientific landscape are essential. Patients should consul Treating Osteoarthritis With a healthcare professional to discuss the risks and benefits of this approach and understand if participation in a clinical trial might be a suitable option. As research progresses, we can anticipate a future where stem cells play a more definitive role in alleviating the burden of OA and improving the lives of millions.
Sources:
Murphy et al., 2008: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2597079/
Whether you need help with marketing, patient financing, legal compliance the team at Rize Up Medical can help.
Let us help you maximize efficiency and profit.

DISCLAIMER: THIS SITE DOES NOT OFFER MEDICAL GUIDANCE. The content available on this website, which includes but is not limited to text, visuals, images, and other materials, is solely for informational purposes. None of the resources provided here should replace expert medical counsel, diagnosis, or care. It is essential to consult your doctor or a competent healthcare professional if you have concerns about any medical conditions or treatments, prior to beginning a new healthcare routine. Do not disregard expert medical recommendations or postpone seeking assistance due to information found on this site.